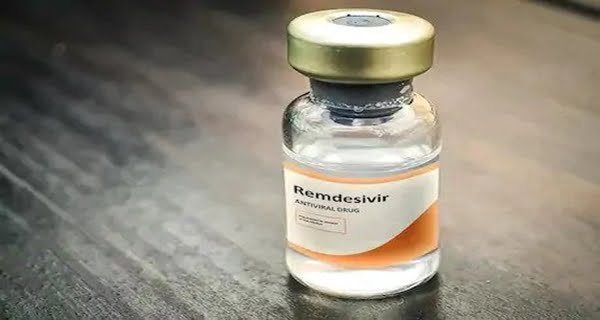
New Delhi: Drugmaker Mylan NV said on Monday that it has received approval to launch a generic version of Remdesivir. For the treatment of Covid-19, Gilead Sciences first launched the drug Remedesivir. Mylan NV said that after the approval, this drug will sell 100 mg vial in India at a price of Rs 4,800. Please tell that India has reached the third place in the case of Coronavirus cases in India.
The Drug Controller General of India (DCGI) has approved the drug Remedesvir of Mylan NV. The drugmaker has given the drug the brand name Desrem. This medicine will be used in case of emergency for Kovid-19 patients. The company has given information about this by issuing a statement.
Two other companies have already launched medicine
Prior to this, two Indian companies have already launched the generic version of this drug. Mylan has now become the third company. Mylan was earlier launched by Cipla Ltd. and Hetero Labs Ltd. Cipla named Cipremi this drug for Rs 5,000 and Hetero priced Covifor at Rs 5,400.
Mylan will produce Remdesvir in India
Mylan NV said that it will make Remedesivir medicine in its own facilities in India. The company said in its statement that the drug has been approved for use for those patients suffering from Covid-19 infection, whose condition is critical. It can be used for adults and children.
Gilead puts the price at $ 2,340 per patient
Please tell that Gilead has put the price of Remedesivir medicine for rich countries at $ 2,340 per patient. The company has agreed to send about half of its total supply to the US in the next three months. After this, concern has increased about the availability of this medicine in other countries around the world.
Remedesiveer has also been approved in Europe
It is noteworthy that it was revealed in the clinical trial of Remdesvir that when this drug is given in intravenous form to Covid-19 patients, they quickly recover from this infection. Since then, the demand for Ramdesvir has increased worldwide. On Friday, this drug has also received conditional approval from the European Commission, after which it can be used in 27 European countries.
Gilead Sciences has tied up with these companies in India
Gilead Sciences has entered into a non-exclusive agreement with Dr Reddy’s Laboratories Limited, Jubilant Life Sciences Limited, Signage International Limited, and Laidus Cadila for India. After this, these companies will be able to produce and sell Remedisvir in India.