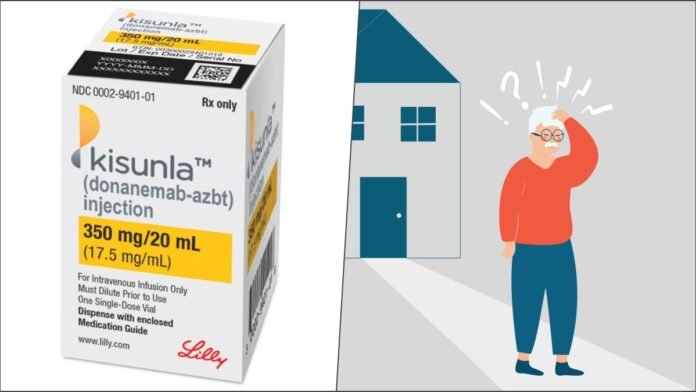
Washington D.C: The U.S. Food and Drug Administration (FDA) has approved Kisunla™, a groundbreaking drug developed by Eli Lilly, to treat early symptomatic Alzheimer’s disease. Let’s delve into the specifics:
- What is Kisunla?
- Kisunla (donanemab-azbt) is a monoclonal antibody infusion administered once a month.
- It targets amyloid plaques in the brain, a hallmark of Alzheimer’s disease.
- The drug’s brand name is Kisunla.
- Who Can Benefit?
- Patients with mild cognitive impairment or those in the early dementia stage are eligible.
- The FDA’s decision is based on a pivotal Phase 3 study involving 1,700 participants.
- Kisunla slowed cognitive and functional decline by up to 35% compared to a placebo over 18 months.
- It also reduced the risk of progressing to the next clinical stage by up to 39%.
- How Does It Work?
- Kisunla removes amyloid plaques from the brain, preserving memory and thinking abilities.
- Patients receive monthly infusions lasting around 30 minutes.
- Nearly half of the study participants completed their treatment within 12 months.
- Why Is This Significant?
- Kisunla is the second drug of its kind approved by the FDA, following Leqembi.
- Alzheimer’s affects 6.7 million Americans aged 65 and older, with projections reaching 13.8 million by 2060.
- Having multiple treatment options is a major advancement for patients and their families.
- Safety Considerations
- Like other drugs in its class, Kisunla has potential side effects, including brain swelling and bleeding.
- However, most cases identified in trials were mild.
- The drug’s approval is a milestone in Alzheimer’s research.
- Alzheimer’s Association’s Response
- The Alzheimer’s Association hails this as real progress.
- Approval of Kisunla provides more options and greater hope for those affected by this devastating disease.
Kisunla represents a significant step forward in Alzheimer’s treatment, offering hope to millions of patients and their loved ones.
Advertisement