Hyderabad: Corona cases are spreading all over the world including China. Even though there is a vaccine to avoid this disease, after becoming positive, no effective medicine has been made in the treatment. But now there is no need to worry. Now its medicine has been made and the World Health Organization has also approved it.
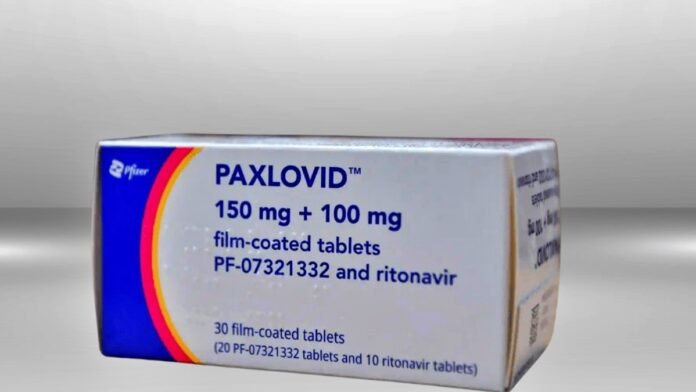
The pharmaceutical company Hetero on Monday announced approval under the World Health Organization (WHO) Pre-Qualified Medicines Program (WHO-PQ) for a generic version of its oral COVID-19 drug Nimrelvir. The company said in a press release that ‘Pfizer Covid-19 oral antiviral drug ‘Paxlovid has received initial approval for the first time.
The WHO has made a strong recommendation for nimatrelvir and ritonavir to be administered to patients with mild to moderate coronavirus infection who are at high risk of hospitalization, the release said. Such patients may be either elderly, their immunity may be low or they may not have been vaccinated.
Nirmacom, a combined pack provided by Hetero, contains 150 mg of Nirmatelvir (two tablets) and 100 mg of Ritonavir (one tablet). This medicine is available only on the advice of a doctor and should be taken as soon as possible after getting infected and within five days from the onset of symptoms. Nirmacom will be manufactured at Hetero’s units in India, the release said.

Vamsi Krishna Bandi, Managing Director, of Hetero Group of Companies, said, “Getting the initial WHO approval for Nirmacom is an important step in the fight against COVID-19, as it will help us increase access to this important innovative antiretroviral drug. We are committed to making Nirmacom available at an affordable cost in 95 LMICs (Low and Middle-Income Countries) in India soon.
About PAXLOVID (nirmatrelvir [PF-07321332] tablets and ritonavir tablets)
PAXLOVID is a SARS-CoV-2 main protease (Mpro) inhibitor (also known as SARS-CoV-2 3CL protease inhibitor) therapy. It was developed to be administered orally so that it can be prescribed early after infection, potentially helping patients avoid severe illness (which can lead to hospitalization and death). Nirmatrelvir [PF-07321332], which originated in Pfizer laboratories, is designed to block the activity of the Mpro, an enzyme that the coronavirus needs to replicate. Co-administration with a low dose of ritonavir helps slow the metabolism, or breakdown, of nirmatrelvir in order for it to remain active in the body for longer periods of time at higher concentrations to help combat the virus.