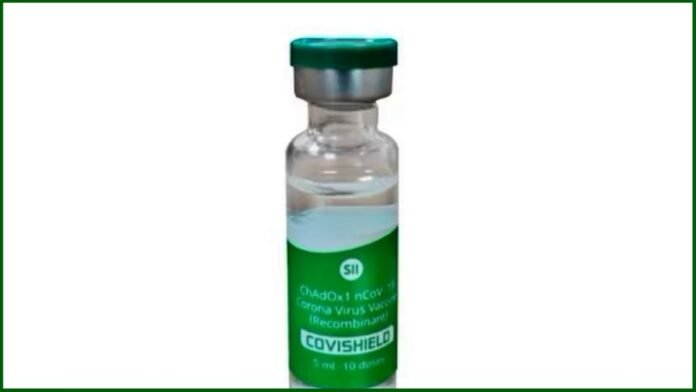
New Delhi: In a significant development, AstraZeneca, the British pharmaceutical giant, has announced a global withdrawal of its COVID-19 vaccine, following admissions in court regarding various side effects. The vaccine, known as Covishield in India and Vaxzevria in Europe, was developed in collaboration with Oxford University and has been a cornerstone in the fight against the coronavirus pandemic.
Serum Institute of India Ceases Production of Covishield
The Serum Institute of India (SII), which had been producing the AstraZeneca vaccine under the brand name Covishield, has also ceased production and supply of the vaccine as of December 2021. The decision comes in the wake of declining demand for the vaccine and the emergence of new coronavirus variants.
Details on the Vaccine Withdrawal
AstraZeneca’s decision to recall the vaccine globally is attributed to the surplus availability of updated vaccines since the pandemic’s onset. The company has initiated the withdrawal process due to the presence of several other vaccines in the market, leading to a decrease in sales.
Serum Institute’s Commitment to Transparency
In response to the vaccine’s side effects, the Serum Institute has emphasized its commitment to transparency and safety. The institute reported all rare to very rare side effects, including blood clotting and low platelet counts, on the vaccine’s packaging as early as 2021. The SII spokesperson stated, “With India achieving high vaccination rates in 2021 and 2022, coupled with the emergence of new mutant variant strains, the demand for previous vaccines diminished significantly. Consequently, since December 2021, we have stopped the manufacturing and supply of additional doses of Covishield.”
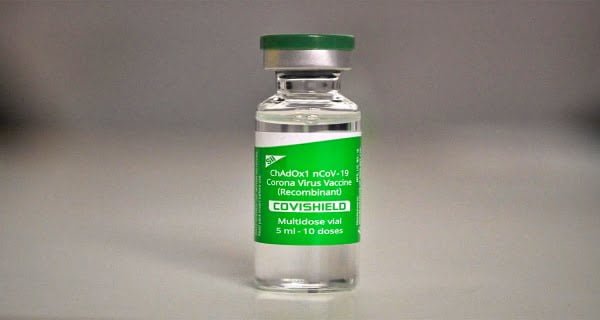
The Future of Covid-19 Vaccines
As the world adapts to the evolving pandemic, pharmaceutical companies are shifting their focus to newer vaccine options that address the latest virus variants. The global withdrawal of AstraZeneca’s vaccine marks a turning point in the ongoing battle against COVID-19, as the world gears up for the next phase of vaccination strategies.