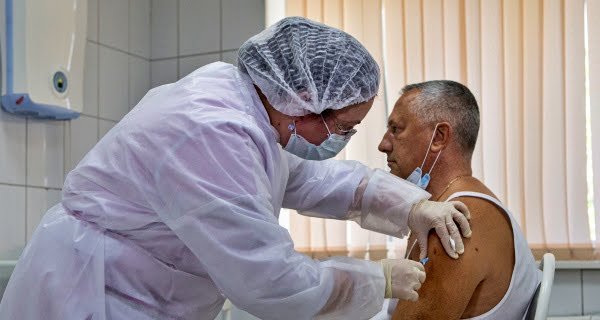
Moscow / Washington: There has been a mixed reaction of people to the Covid-19 vaccine ‘Sputnik V vaccine’ developed in Russia. Health workers and teachers are being vaccinated in the first phase, but people are not coming to Moscow to vaccinate at many clinics. On the other hand, the US has also started the work of Pfizer Coronavirus Vaccine, but getting the elderly people to get vaccinated is proving challenging for the US Department of Health. The survey has also revealed that more than 70% of the elderly are concerned about the vaccine being safe.
Let us know that after the Russian government and the media approved the ‘Sputnik V’ vaccine on August 11, it was described as a huge achievement. But, there is not a very enthusiastic response among the common people about the vaccine and many people are raising doubts about its effective and safe. Russia has also faced criticism for not completing all phases of the experimental test. Experts from home and abroad also warned against widespread use of the vaccine till its completion. However, the administration, ignoring suggestions, started giving vaccines to at-risk groups, including health workers working on the advance fronts. Alexander Gintesburg, head of the Gamalaya Institute that developed the vaccine, said last week that more than one and a half million people in Russia had been given vaccine doses.
People are not coming to the hospital
ICU specialist Alexander Justsepine also took a vaccine dose in Voronezh, about 500 km from Moscow. He said that despite taking the vaccine, he is taking precautions because the study about its effect has not been completed yet. Britain approved Pfizer’s vaccine on December 2. After this, Russia also started vaccination on a large scale due to the possibility of being left behind in the competition for vaccine manufacture. Russia approved the vaccine developed in its country only after clinical trials on a few dozen people. The vaccine makers named it ‘Sputnik V’. In this way, its reference was linked with the first satellite left by the Soviet Union in 1957 during the Cold War.
In the UK, vaccine doses are first given to the elderly, while ‘Sputnik V’ is being given to people between the ages of 18 and 60. Vaccine manufacturers have said that the study showed that the sputum vaccine was 91 percent effective. This result was derived from a study conducted on about 23,000 participants. Whereas, Western countries included more people, people of different backgrounds, ages in the test. Levada Center, an independent survey organization in Russia, conducted an opinion poll in October, in which 59 percent said they would not like to take the vaccine despite being offered it. After talking to some health workers and teachers, it went on to say that they do not want to take the vaccine due to not being tested properly.
The elderly are not ready in America
Pfizer’s Corona vaccine has started to be used in America. Soon Moderna’s vaccine is also going to get regulatory permission. Both vaccines have been claimed to be 95 percent effective and also safe. A member of the Advisory Committee which approved the vaccine had also voted against its use among the elderly. Committee member Dr. Helen Keep Talbot believes that the vaccine has just received emergency use. It has not been tested the way a vaccine is usually tested. If the vaccine is not effective in the elderly or if there are more side effects then there may be panic in the people. However, other experts of the committee are saying that according to the evidence so far, it can be said that the vaccine is safe and no one, including the elderly, should worry about it.
Stigma regarding vaccines in the elderly has increased after Sanofi’s test result. Sanofi and Glaxo Smith Kline had said that the trial results did not go as expected and the vaccine is not proving to be more effective in older people. Therefore, the Sanofi vaccine has been postponed till the end of 2021. However, both Pfizer and Moderna have claimed that their vaccine is completely effective even in the elderly. Nearly 40 percent of deaths due to corona in the US have been from people living in nursing homes. The vaccine has just received emergency permission instead of regular permission and half of Americans are apprehensive. According to a survey by Virginia Commonwealth University, only 46.9 percent of people said that they would definitely take the vaccine. At the same time, if the vaccine gets regular permission, about 60 percent of the people are ready to do its dose.


















































