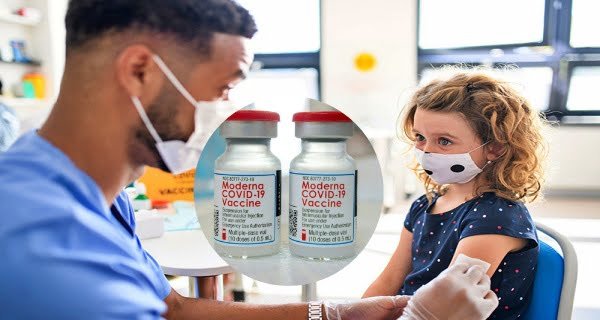
New Delhi: In view of the fears of a third wave of coronavirus worldwide, the process of vaccination has been expedited. Not only the elderly but many countries have now recommended the vaccination of children. Meanwhile, the news of relief is that Europe’s drug regulator has recommended approving the use of Moderna’s Covid vaccine for children aged 12 to 17 years. The European Medicines Agency (EMA) said the use of the vaccine in children will be the same as in people over the age of 18. The EMA said that post-vaccination, antibody responses have been seen in people between the ages of 18 and 25.
The EMA says that vaccination is necessary to protect children from the delta variant of Corona and this modern vaccine will work to strengthen their immunity. For information, let us tell you that two doses of Moderna Vaccine are taken and there is a gap of 4 weeks between the first and second doses.

The European Union is evaluating the application
Dr. Marco Cavallari, head of the vaccine strategy for the European Union’s drug regulator, said the expert committee is currently evaluating the application of Moderna to increase the use of the coronavirus vaccine for children aged 12 to 17 years. He said, we are hopeful that the committee will reach a conclusion by the end of next week, after which it can be approved.
Moderna’s vaccine was approved in January for use in people 18 years of age and older in the European Union of 27 countries. It has also been licensed in countries including the UK, Canada, and the US, but has not yet been used for children. To date, the vaccine made by Pfizer-BioNTech is the only vaccine approved for children under 18 in Europe and North America.