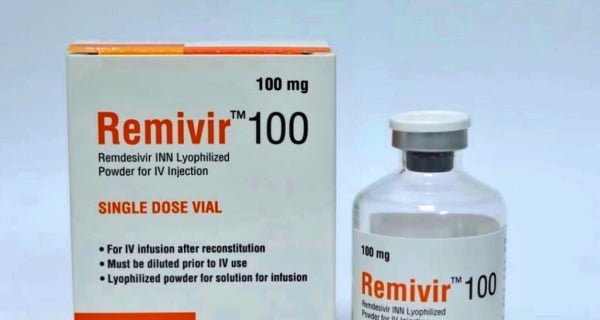
Geneva: The World Health Organization has taken a major step towards the anti-viral drug Remedivir. The WHO has removed Gilead’s Remedesivir from its alleged prequalification list. This was confirmed by Tariq Jasrovich in response to an email from the news agency Reuters. “Yes, we have removed it from PQ,” he said. Earlier on Friday, the WHO had said that Remedesivir should not be used in the treatment of Kovid-19 patients. Because it is not having any significant effect on the patient’s chances of survival.

Significantly, Remedicivir was considered a major weapon in the treatment of corona. Not only that, but Remadesivir was also used to treat U.S. President Donald Trump. Many previous studies have claimed that this drug is reducing the treatment time of patients. Remedesivir was approved in more than 50 countries for the treatment of Covid-19. The United States, the European Union, and other countries temporarily allowed the use of Remedivir. The IWHO’s recommendation on Friday was based on four international random trials. These trials involved 7 thousand of hospitalized patients.
Pfizer sought permission from the U.S. government
Pfizer, one of the leading candidates in the vaccine race, has sought permission from the U.S. government for the emergency use of the drug. Pfizer said its vaccine was 95 percent effective against the coronavirus. The company says allowing emergency use could speed up the process and doses of the coronavirus vaccine could be available by next month.
The Oxford vaccine may arrive by April
The statement of Adar Poonawala of Serum Institute, which is preparing vaccines in India, can bring relief to Indians. Arriving at the Hindustan Times Leadership Summit 2020, Poonawala said on Thursday that the Oxford Covid-19 Vaccine should be available to healthcare personnel by next February. However, the general public can get this vaccine till April. He informed that the cost of two essential doses of vaccine will be up to a maximum of Rs. 1 thousand. However, it all depends on the results of the last trials and the permission from the regulators.