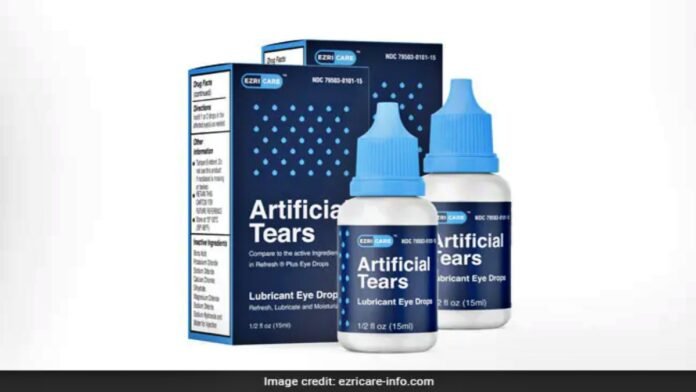
Washington: An Indian company manufacturing eye drops in the US has been embroiled in controversies. Eye infections have claimed many lives in the United States. After this, the Indian company Global Pharma Healthcare recalled eye drops from the market. The US Food and Drug Administration has informed us about this. A person died after using an eye drop manufactured by an Indian company. That’s why the FDA said that the company’s drops have been recalled from the US market.
One died due to excessive bleeding from the eye
A total of 55 cases of the effect of Azricare Drop have been reported. The FDA informed that the recall is being done due to suspected drops manufactured by Chennai-based Global Pharma Healthcare Company. Several cases of infection have been reported in several US states from eye drops of an Indian pharmaceutical company. Therefore, the Centers for Disease Control notified the FDA. In the incidents that have come to light so far, many people have lost their eyesight. Many people get eye infections. One died of excessive bleeding from the eye. This drop is used to prevent burning and dryness of the eyes.

Eye drops back from the market
In this matter, Global Pharma Healthcare has issued a statement on its website. Aru Pharma Inc and Delsum Pharma the distributors of this product have been instructed to withdraw the eye drops from the market and stop their use. The company said in a statement that if you feel any problem after using this medicine, you should contact your doctor. Artificial Tears Lubricant Eye Drops are used to relieve irritation and dryness in the eyes. Global Pharma Healthcare is practiced in various countries in South East Asia, Central America, and Africa.


















































