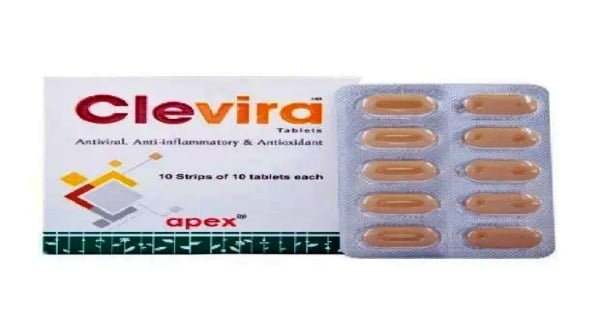
Thiruvananthapuram: Marking a landmark development in the fight against COVID-19 Pandemic in India and around the globe, Apex Laboratories Private Limited, a Chennai-based Pharmaceutical manufacturer and exporter known for Research, Innovation, and quality medicines for more than four decades, on Friday announced the approval of Antiviral drug CleVira as a supporting measure for Mild to Moderate condition of COVID-19.
Apex Laboratories Private Limited has received approval from Government of India (Ministry of AYUSH) regulators, for their Anti-Viral Drug CleVira as an additional indication as a supporting measure for Mild to Moderate condition of COVID-19 making it first of its kind approval in India through various stages of scrutiny at CCRAS (The Central Council for Research in Ayurvedic Sciences) and Inter-Disciplinary Technical Review Committee (ITRC), a-12-member technical committee constituted by Ministry of AYUSH and headed by Dr. S K Maulik Former Professor Department of Pharmacology AIIMS.
CleVira is the brainchild of Apex’s R&D Center based on proven scientific evidence and was launched in the Indian market during the recent outbreak of the Dengue epidemic and associated mortality in 2017.
CleVira is extensively studied for its safety in animal models (Wistar rats) and efficacy in Human subjects in Phase II and III clinical trials. CleVira is an approved Antiviral formulation for the treatment of various viral infections including viral fever associated with or without thrombocytopenia. CleVira has proven its efficacy as Analgesic, Antipyretic, and reversal of thrombocytopenia apart from its Antiviral property.

A phase III Clinical trial was carried out in Government Medical College Omandurar Govt. Estate Chennai, based on Government approval from The State Government of Tamil Nadu.
The trial outcomes revealed that CleVira has shown an 86% recovery rate on the 5th day of treatment in Mild to Moderate COVID-19. 100% recovery rate noticed on the 10th day of treatment. Clinical recovery from all signs and symptoms was registered in 4 days. CleVira is proven to be safe on Kidney and Liver parameters.
The study outcomes were placed before the Government of Tamil Nadu, the Indian Council of Medical Research (ICMR), and the Ministry of AYUSH in 2020.
After various stages of scrutiny and deliberations before various technical review committees, Government of India (Ministry of AYUSH) regulators have granted approval for CleVira as a supporting measure for Mild to Moderate condition of COVID-19.
CleVira is effective when taken orally and the dosage is one tablet twice daily after food for 14 days.
Apex Laboratories Private Limited is a more than 4 decades old Pharmaceutical manufacturer and exporter based in Chennai India, known for its flagship brands such as Zincovit, P range of Paracetamol, and a whole range of branded formulations including dermatology, having a global presence in over 30 countries.
Research, Innovation, and quality are the guiding principles of Apex. It is ranked among the top 50 Indian pharmaceutical companies. For more information visit www.apexlab.com