Key Points:
- Death toll rises to 18 children across Madhya Pradesh and Rajasthan due to contaminated Coldrif cough syrup
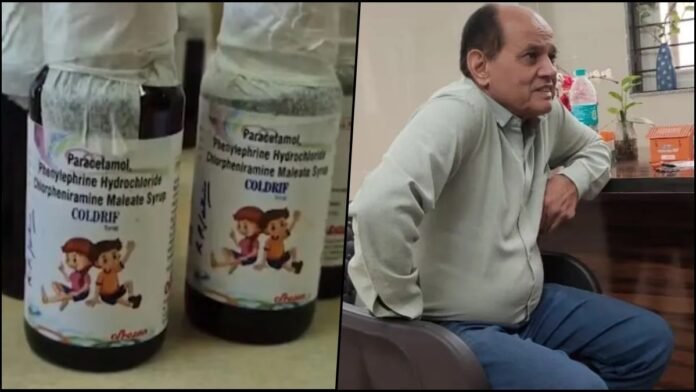
- Dr. Praveen Soni arrested Saturday night for prescribing the toxic medication to children
- Laboratory tests reveal 48.6% diethylene glycol concentration in syrup, far exceeding 1% permissible limit
- Madhya Pradesh government bans Coldrif syrup statewide, suspends all Sresan Pharmaceuticals products
- FIR filed against doctor and Tamil Nadu-based manufacturer under BNS sections 105, 276, and Drugs and Cosmetics Act
- Congress MLA Kamal Nath demands ₹50 lakh compensation, calls incident “man-made tragedy”
- Tamil Nadu factory operations suspended, nationwide drug quality inspections ordered at 19 manufacturing sites
New Delhi: The devastating tragedy that began in Chhindwara, Madhya Pradesh, has now claimed 18 innocent lives across two states, sending shockwaves through the country’s healthcare system. What started as routine cold and fever complaints in early September transformed into a nightmare for families as children developed acute kidney failure after consuming the contaminated Coldrif cough syrup. Of the total fatalities, 14 deaths occurred in Chhindwara district alone, while four additional deaths were reported from Rajasthan’s Sikar, Bharatpur, and Churu districts, all linked to the same toxic batch.
Doctor Arrested After Damning Investigation
In a significant breakthrough, Madhya Pradesh Police arrested pediatrician Dr. Praveen Soni late Saturday night near Rajpal Chowk in the Kotwali Police Station area of Chhindwara. The arrest followed a formal complaint filed by Dr. Ankit Sahlam, Block Medical Officer of Parasia Community Health Centre, after investigations revealed that Dr. Soni had prescribed the contaminated syrup to most of the affected children at his clinic in Parasia. Police registered a case under stringent provisions including BNS Sections 105 (culpable homicide not amounting to murder) and 276 (adulteration of drugs), along with Section 27(A) of the Drugs and Cosmetics Act, which carries punishment ranging from ten years imprisonment to life imprisonment.
Shocking Toxicity Levels Uncovered
The most alarming revelation came from laboratory analysis conducted by Tamil Nadu’s Food and Drug Administration, which collected samples directly from Sresan Pharmaceuticals’ manufacturing facility in Kanchipuram. The test results, shared on October 3, 2025, revealed that Coldrif syrup contained a staggering 48.6% concentration of diethylene glycol (DEG), a highly toxic industrial solvent used in antifreeze, paints, and brake fluids. Chhindwara District Magistrate Harendra Narayan described the findings as “disturbing,” stating that the permissible limit should be within 1%, making the detected levels dangerously toxic even for adults, let alone children.
Diethylene glycol is nephrotoxic, meaning it specifically damages kidneys and produces diglycolic acid as its main toxic by-product, leading to acute kidney injury (AKI) characterized by rapid kidney failure. Medical experts noted that the children’s deterioration was alarmingly swift, with kidney biopsies revealing damage far beyond normal levels, confirming DEG poisoning as the cause of death.
Swift Government Action and Statewide Ban
Chief Minister Mohan Yadav responded swiftly to the crisis, announcing an immediate and complete ban on the sale and distribution of Coldrif syrup across Madhya Pradesh on October 3, 2025. The ban was extended to all other pharmaceutical products manufactured by Sresan Pharmaceuticals, with orders to seal existing stocks until further notice. In a statement on social media platform X, CM Yadav declared the deaths “extremely tragic” and assured that “the culprits will not be spared at any cost”.
The state government also announced ₹4 lakh compensation for each affected family and pledged to bear all medical treatment costs for children still undergoing treatment. Additionally, the Chief Minister requested the Tamil Nadu government to conduct a thorough investigation into Sresan Pharmaceuticals’ Kanchipuram facility, where the contaminated batch was manufactured.
Tamil Nadu Takes Action, Central Government Steps In
Following confirmation of the contamination, Tamil Nadu authorities banned the sale of Coldrif syrup across the state from October 1, 2025, and ordered the suspension of production at Sresan Pharmaceuticals’ Kanchipuram facility pending further investigation. The syrup had been distributed to Madhya Pradesh, Rajasthan, and Puducherry before the ban was imposed.
At the national level, the Union Health Ministry issued an advisory on October 3 stating that cough and cold medications should not be prescribed or dispensed to children below two years of age. The Central Drugs Standard Control Organisation (CDSCO) has ordered risk-based inspections at 19 drug manufacturing sites across six states to uncover quality control lapses. A multidisciplinary investigation team comprising experts from the National Institute of Virology (NIV), Indian Council of Medical Research (ICMR), National Environmental Engineering Research Institute (NEERI), CDSCO, and AIIMS Nagpur continues to probe the cause of deaths.
Political Firestorm Erupts Over Compensation
The tragedy has sparked intense political debate, with senior Congress leader and Chhindwara MLA Kamal Nath sharply criticizing the state government’s response. Describing the incident as a “man-made tragedy,” Nath condemned the ₹4 lakh compensation announced by Chief Minister Yadav as grossly inadequate and demanded ₹50 lakh financial assistance for each affected family. He further called on the government to bear the complete cost of treatment for all sick children and launch a comprehensive campaign against counterfeit and poisonous medicines being sold across Madhya Pradesh to prevent such incidents in the future.
Pattern of Devastating Illness
According to distraught families, the affected children first complained of cold and mild fever in early September 2025. After being prescribed routine medication including cough syrups, they initially appeared to recover. However, within days, symptoms returned with alarming intensity, followed by a sudden and severe decrease in urine output a critical sign of kidney failure. Their condition rapidly deteriorated into full-blown kidney infections, ultimately leading to death despite medical intervention. Four renal biopsies conducted on deceased children conclusively revealed the presence of diethylene glycol contamination, confirming the poisoning.
Ongoing Investigations and Quality Control Concerns
The tragedy has exposed serious gaps in India’s pharmaceutical quality control system and raised urgent questions about drug safety standards. Interestingly, while six samples collected by CDSCO from Madhya Pradesh’s Chhindwara tested negative for DEG and ethylene glycol contamination, samples taken directly from the Tamil Nadu manufacturing facility confirmed toxic levels, suggesting batch-specific contamination at the source. Authorities continue to investigate how such dangerously contaminated medication received manufacturing approval and distribution clearance, putting thousands of children at risk across multiple states.







































